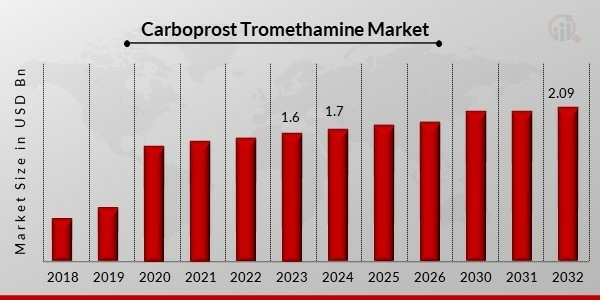
Carboprost Tromethamine Market Overview
The Carboprost Tromethamine market is gaining notable momentum as healthcare systems globally emphasize maternal health, particularly postpartum care and emergency obstetrics. Carboprost Tromethamine, a synthetic prostaglandin analogue, is widely used in the management of postpartum hemorrhage (PPH) and for second-trimester pregnancy termination. It is highly effective in inducing uterine contractions, controlling uterine bleeding, and facilitating complete uterine evacuation. With the rising awareness of women's reproductive health, increasing maternal morbidity rates in certain regions, and growing access to emergency obstetric care, the demand for Carboprost Tromethamine is expected to grow significantly over the forecast period.
Market Overview
Carboprost Tromethamine has long been a critical drug in the treatment of postpartum hemorrhage—a leading cause of maternal mortality worldwide. Its efficacy in inducing strong uterine contractions makes it especially useful when other medications such as oxytocin or misoprostol fail. The increasing use of Carboprost Tromethamine in hospitals, emergency obstetric centers, and clinics is a key driver of the market. Additionally, its role in second-trimester abortion procedures, particularly in cases of incomplete miscarriage or fetal anomalies, is contributing to market expansion.
The global Carboprost Tromethamine market is showing steady growth, supported by increased demand in both developed and developing economies. Advances in drug formulations, better access to essential medicines, and rising emphasis on women's health policies are further fueling the market. The growing healthcare expenditure, particularly in maternal health, and improvements in healthcare infrastructure across low- and middle-income countries are also significant contributors.
Key Market Segments
The Carboprost Tromethamine market can be segmented based on formulation type, application, distribution channel, and region.
By Formulation Type:
-
Injectable Solution
-
Ampoules
-
Vials
Injectable formulations dominate the market due to the urgent nature of the conditions treated with Carboprost Tromethamine, such as uncontrolled postpartum bleeding. Ampoules and vials are commonly used for single-use purposes in hospitals and maternity wards.
By Application:
-
Postpartum Hemorrhage Management
-
Second-Trimester Abortion
-
Uterine Atony
-
Incomplete Miscarriage
-
Other Gynecological Indications
Postpartum hemorrhage management accounts for the largest market share. However, second-trimester abortion and uterine atony management are emerging as significant applications due to rising demand for safe, medically supervised reproductive health solutions.
By Distribution Channel:
-
Hospital Pharmacies
-
Retail Pharmacies
-
Online Pharmacies
Hospital pharmacies dominate the market due to the critical and time-sensitive nature of Carboprost Tromethamine’s applications. However, with the expansion of telemedicine and e-commerce platforms, online pharmacies are gradually increasing their market share.
By Region:
-
North America
-
Europe
-
Asia Pacific
-
Latin America
-
Middle East & Africa
Industry Latest News
The Carboprost Tromethamine market has seen several developments in recent years, particularly around policy changes and healthcare access. In 2024, global health organizations launched new maternal care guidelines reinforcing the use of Carboprost Tromethamine for the management of postpartum hemorrhage in emergency care settings. This led to increased procurement by public health agencies in low-income regions, especially across Africa and Southeast Asia.
Pharmaceutical companies are also investing in the development of more stable formulations with longer shelf life, making the drug more accessible in remote areas without cold chain storage facilities. Some manufacturers have expanded production capacity in response to growing demand from government tenders and global health initiatives.
There has also been a focus on educating healthcare professionals about proper administration techniques and contraindications, especially in resource-limited settings where training gaps previously contributed to underutilization.
Key Companies
Several pharmaceutical companies are involved in the manufacture and distribution of Carboprost Tromethamine, supplying it to both public health agencies and private healthcare providers. These companies focus on high-quality, cost-effective formulations to meet global maternal health needs.
Key players in the Carboprost Tromethamine market include:
-
Pfizer Inc.
-
Hikma Pharmaceuticals
-
Bionpharma Inc.
-
JHP Pharmaceuticals (Part of Par Pharmaceutical)
-
LGM Pharma
-
Amneal Pharmaceuticals
-
Teva Pharmaceutical Industries Ltd.
-
Endo International plc
-
Fresenius Kabi
-
Troikaa Pharmaceuticals
These companies compete based on pricing, product availability, compliance with international regulatory standards, and the ability to supply in bulk for healthcare procurement contracts. Pfizer and Hikma are among the most prominent players due to their global reach and established supply chains in essential medicines.
Market Drivers
Several key drivers are propelling the growth of the Carboprost Tromethamine market:
1. Rising Incidence of Postpartum Hemorrhage:
With postpartum hemorrhage remaining a leading cause of maternal death in many countries, the urgent need for effective uterotonic agents like Carboprost Tromethamine is driving market demand.
2. Global Focus on Maternal Health:
International organizations and national health ministries are increasingly investing in maternal health programs, particularly those aimed at reducing maternal mortality. These programs often include Carboprost Tromethamine in their essential drug lists.
3. Expanding Access to Emergency Obstetric Care:
As more hospitals and rural healthcare centers gain access to emergency obstetric care resources, the use of injectable uterotonics like Carboprost Tromethamine is expected to rise.
4. Supportive Regulatory Policies:
Regulatory bodies across regions are fast-tracking approvals for maternal health drugs, and several countries have streamlined procurement processes for essential medications, facilitating faster market access.
5. Technological Advancements in Drug Formulation:
Ongoing research into thermally stable formulations is expanding the availability of Carboprost Tromethamine in regions with limited cold storage facilities, thereby increasing market penetration.
6. Increased Awareness and Education:
Educational campaigns and healthcare training initiatives have raised awareness among healthcare professionals regarding the timely and correct use of Carboprost Tromethamine, improving its clinical uptake.
Browse In-depth Market Research Report ➤➤➤ https://www.marketresearchfuture.com/reports/carboprost-tromethamine-market-22113
Regional Insights
North America dominates the Carboprost Tromethamine market, supported by advanced healthcare infrastructure, high awareness levels, and the strong presence of leading pharmaceutical companies. The U.S. holds a significant share due to high rates of cesarean deliveries and rising awareness of postpartum hemorrhage management protocols.
Europe is another strong market, with countries such as the UK, Germany, and France leading the adoption of evidence-based maternal care practices. National health systems in these countries often include Carboprost Tromethamine in their obstetric treatment protocols.
Asia Pacific is witnessing rapid growth in the Carboprost Tromethamine market due to increased investments in maternal health, rising birth rates, and higher healthcare spending. Countries like India, China, and Indonesia are enhancing their rural health infrastructure, which is driving greater demand for essential obstetric drugs.
Latin America and the Middle East & Africa are gradually emerging as important markets, backed by international maternal health programs and aid from global health organizations. These regions often rely on bulk drug procurement through government contracts and international donors to supply hospitals and health centers.
Conclusion
The Carboprost Tromethamine market is positioned for steady growth as global efforts to reduce maternal mortality and improve access to emergency obstetric care intensify. With a strong focus on evidence-based maternal healthcare, rising incidences of postpartum hemorrhage, and increasing acceptance of pharmaceutical interventions in reproductive health, the demand for Carboprost Tromethamine is expected to rise significantly in the coming years.
Explore MRFR’s Related Ongoing Coverage In Healthcare Domain:
Retail Clinic Market Size, Trends, Growth Report 2035 | MRFR
Sacral Nerve Stimulation Market Size, Growth Outlook 2035
Self Injection Device Market Size, Growth Report 2035 | MRFR
Sexually Transmitted Diseases Diagnostic Market Report 2035 | MRFR
Sickle Cell Disease Market Size, Trends, Growth Report 2034 | MRFR